Has anyone that is taking Otezla gotten one or multiple. Pregnant women should be advised of the potential risk of fetal loss.
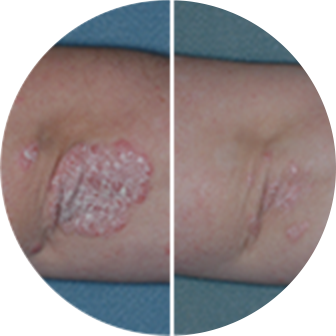 See A List Of Side Effects Of Otezla Apremilast
See A List Of Side Effects Of Otezla Apremilast
Upper respiratory tract infection 39 18.

Otezla upper respiratory infection. The Otezla group was higher but the numbers are small enough that its uncertain. Otezla is a prescription medicine approved for the treatment of adult patients. This may lead to a reduction in chemicals that cause inflammation in the body.
Upper respiratory tract infection 39 18. Upper respiratory tract infection 39 18. My coughcolds just take longer to clear up.
Over 112 days 24 of the placebo group and 45 of the Otezla group got upper respiratory infections. Side effects of Otezla include diarrhea nausea vomiting upper respiratory tract infection runny nose sneezing or congestion abdominal pain tension headache and headache. Upper respiratory tract infection is found among people who take Otezla especially for people who are female 60 old have been taking the drug for 1 - 6 months.
Maybe there wasnt much going around at the time. The most common adverse reactions leading to discontinuation for subjects taking Otezla were nausea 16 diarrhea 10 and headache 08. Otezla is a systemic therapy different from non-systemic topical treatments like creams or shampoos that are applied directly to the scalp.
Otezla works by reducing production of an enzyme known as phosphodiesterase 4 PDE4. But I have always ridden them out without discontinuing Otezla. Otezla apremilast is a prescription medicine approved for the treatment of adult patients with moderate to severe plaque psoriasis for whom phototherapy or systemic therapy is appropriate.
Otezla is meant to be a long-term treatment for psoriatic arthritis plaque psoriasis or canker sores from Behçets disease. Diarrhea nausea and upper respiratory tract infection were the most commonly reported adverse reactions. Upper respiratory tract infection 39 18.
Following the 5-day titration the. Upper respiratory tract infection 39 18. Otezla is proven to help get clearer skin on the scalp.
Adverse reactions reported in at least 2 of patients taking Otezla that occurred at a frequency at least 1 higher than that observed in patients taking placebo for up to 16 weeks after the initial 5-day titration were Otezla placebo. The reason is that the one time I stopped Otezla the psoriasis came back with a vengeance. 21 rows Diarrhea nausea and upper respiratory tract infection were the most commonly reported.
Thats because Otezla is a medication that treats beneath the skinits a pill for moderate to severe scalp psoriasis. Adverse reactions reported in at least 2 of patients taking Otezla that occurred at a frequency at least 1 higher than that observed in patients taking placebo for up to 16 weeks after the initial 5-day titration were Otezla placebo. The phase IV clinical study is created by eHealthMe based on reports of 108085 people who have side effects when taking Otezla from the FDA and is updated regularly.
2 DOSAGE AND ADMINISTRATION 21 Dosage in Psoriatic Arthritis Psoriasis and Behçets Disease The recommended initial dosage titration of OTEZLA from Day 1 to Day 5 is shown in Table 1. Those numbers seem low to me though the trial was only 112 days. Otezla is a prescription medicine approved for the treatment of adult patients with active psoriatic arthritis.
Adverse reactions reported in at least 2 of patients taking Otezla that occurred at a frequency at least 1 higher than that observed in patients taking placebo for up to 16 weeks after the initial 5-day titration were Otezla placebo. When youre considering taking this drug its helpful to know. Adverse reactions reported in at least 2 of patients taking Otezla that occurred at a frequency at least 1 higher than that observed in patients taking placebo for up to 16 weeks after the initial 5-day titration were Otezla placebo.
Adverse reactions reported in at least 2 of patients taking Otezla that occurred at a frequency at least 1 higher than that observed in patients taking placebo for up to 16 weeks after the initial 5-day titration were Otezla placebo. One of the side effects of taking Otezla is Upper Respiratory Infections. And if so how long were you taking it before it happened.
Common side effects of Otezla include diarrhea nausea headache and upper respiratory tract infections. Thats a known side effect of Otezla that infections are harder to get rid off because it suppresses the immune system. These are not all the possible side effects with Otezla.
OTEZLA is indicated for the treatment of adult patients with oral ulcers associated with Behçets Disease.
